Introduction to Chemical Bonding
Introduction to Chemical Bonding: Overview
This Topic covers sub-topics such as Chemical Bond, Valence Electrons, Types of Chemical Bonds, Duplet and, Kossel-Lewis Theory
Important Questions on Introduction to Chemical Bonding
On what basis duplet set of arrangement of electrons can be defined?
Define duplet set of arrangement of electrons.
How do you define duplet set of arrangement of electrons?
How are sodium and chlorine atoms attain octet configuration in the formation of sodium chloride.
What are valence electrons? Write the number of valence electrons in the chlorine and carbon.
What do you mean by valence electrons?
The electrons present in the outermost shell of an atom are called valence electrons.
Regarding the structure of the cyanamide ion, pick out the wrong statement:
Use the letters only written in the periodic table given below to answer the questions that follow
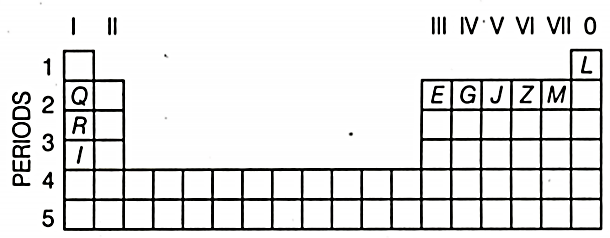
(i) State the number of valence electrons in atom .
(ii) Which element forms ions with a single negative charge?
(iii) Out of and which one is more reactive?
(iv) Which element has its electrons arranged in four shells?
Define valence electrons.
Define valence electrons.
How many valence electrons are present in the element of third period and third group?
Consider the following statements related to ozone (O3) and select the correct ones
I. Bond order between oxygen atoms is 1.5
II. In Lewis structure of ozone all three oxygen atoms carry zero formal charge
III. In Lewis structure of ozone, one of the three oxygen atom carry +1 unit formal charge
Electron deficient molecule is
What is the nature of chemical bonding between and
Oxygen atom and oxide ion have the
Number of electrons in the valence orbit of nitrogen in an ammonia molecule is:
An atom of an element has electrons in its outermost orbit and that of has electrons in its outermost orbit. The formula of the compound between these two will be
The electronic theory of bonding was proposed by
Which of the following bond is the weakest?
